

The non-metals have no metallic luster, and do not reflect light.
The non-metals can be gases, such as oxygen and solids, such as carbon. As opposed to metals, non-metallic elements are very brittle. Non-metals are not able to conduct electricity or heat very well. Non-metals are the elements in groups 14-16 of the periodic table. Some of the metalloids, such as silicon and germanium, are semi-conductors. Metalloids have properties of both metals and non-metals. Metalloids are the elements found between the boundary that distinguishes metals from non-metals. They have oxidation numbers of +3, ±4, and -3. All of these elements are solid, have a relatively high density, and are opaque. These elements, unlike the transition elements, do not exhibit variable oxidation states, and their valence electrons are only present in their outer shell. While these elements are ductile and malleable, they are not the same as the transition elements. The "other metals" elements are located in groups 13, 14, and 15. This is why they often exhibit several common oxidation states. Their valence electrons are present in more than one shell. The 38 elements in groups 3 through 12 of the periodic table are called "transition metals." As with all metals, the transition elements are both ductile and malleable, and conduct electricity and heat.
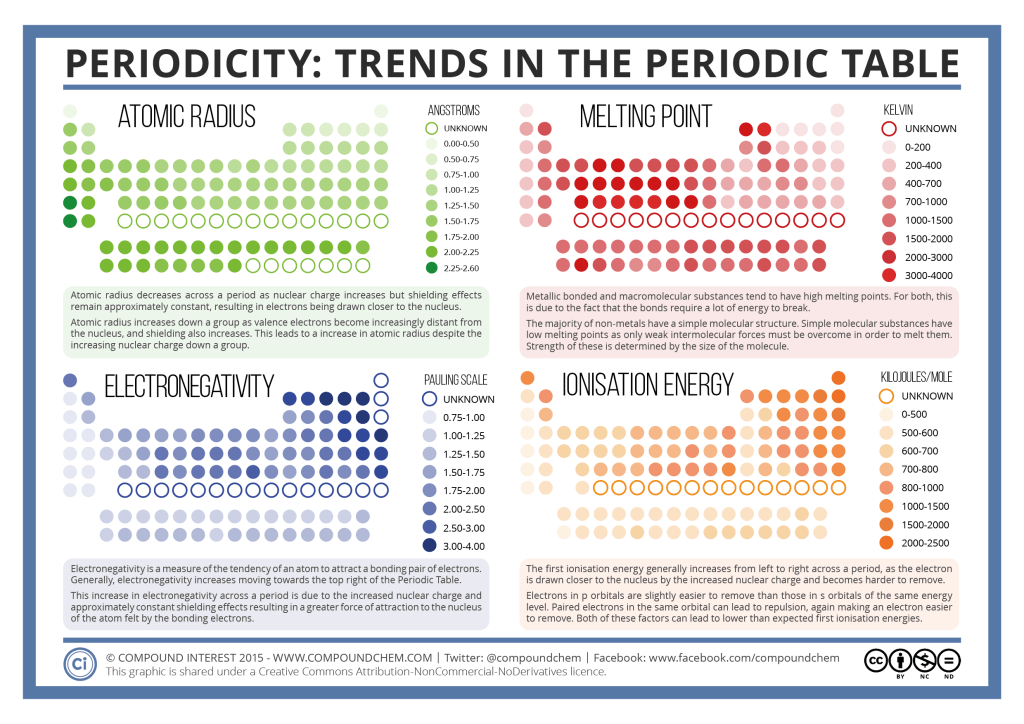
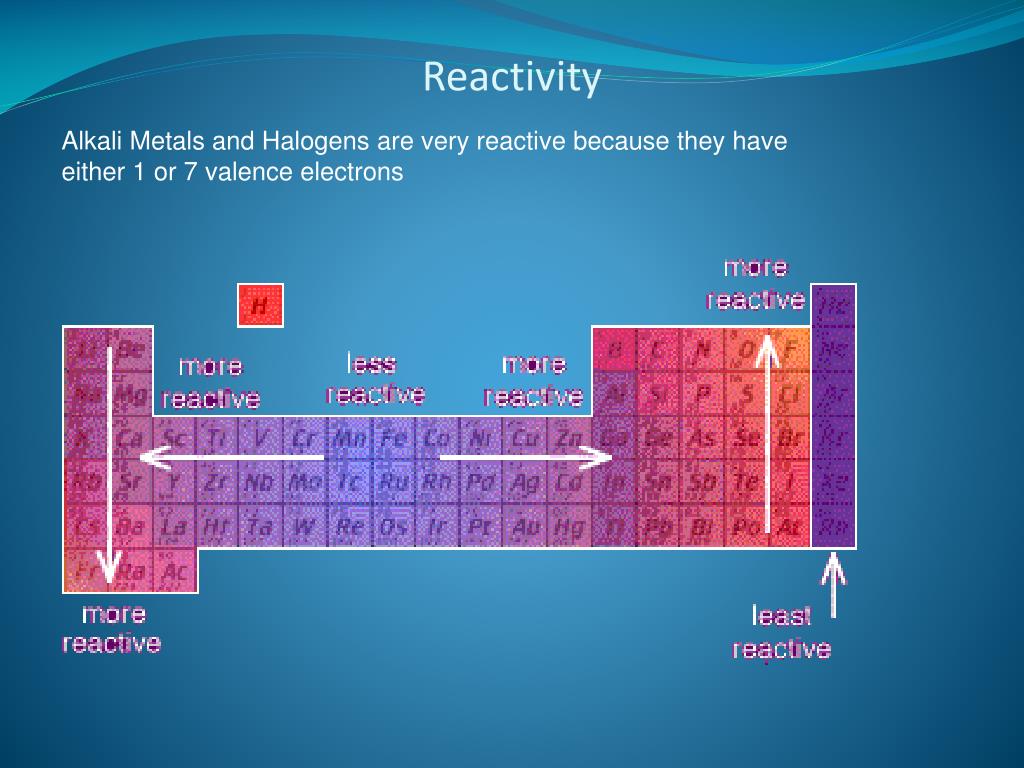
All alkaline earth elements have an oxidation number of +2, making them very reactive. The alkaline earth elements are metallic elements found in the second group of the periodic table. The alkali metals are softer than most other metals. As with all metals, the alkali metals are malleable, ductile, and are good conductors of heat and electricity. Therefore, they are ready to lose that one electron in ionic bonding with other elements. These metals have only one electron in their outer shell. The alkali metals, found in group 1 of the periodic table, are highly reactive metals that do not occur freely in nature. For our purposes we will define the following ten families: The Periodic table can also be divided into several families of elements each having similar properties. Elements of each group had similar properties. The modern one states that the properties vary with atomic number, not weight.Įlements in Mendeleev's table were arranged in rows called periods. His periodic law states that the chemical and physical properties of the elements vary in a periodic way with their atomic weights. He observed that many elements had similar properties, and that they occur periodically. Mendeleev created the first periodic table based on atomic weight. They both created similar periodic tables only a few months apart in 1869. The modern periodic table, based on atomic number and electron configuration, was created primarily by a Russian chemist, Dmitri Ivanovich Mendeleev, and a German physicist, Julius Lothar Meyer, both working independently. These electrons screen or shield the outer electrons from the nuclear charge.Chemistry and Periodic Table Applications This is because of the screening effect of the filled inner electron levels. The greater attraction between the increased number of protons (increased nuclear charge) and electrons, pulls the electrons closer together, hence the smaller size.Īs you move down a group in the periodic table, the covalent radius increases. The covalent radius (a measure of how large individual atoms are) shows different trends if you are moving across a period or down a group.Ī comparison of the relative covalent radii of atoms is shown in the diagram below.Īcross a period from left to right, the covalent radius decreases.Īs you move from left to right across the periodic table, atoms have more electrons in their outer energy level and more protons in their nucleus. Patterns and trends in the periodic tableĬhemists observe patterns in different properties of elements as they are arranged in the periodic table.


 0 kommentar(er)
0 kommentar(er)
